butane bottles, regulators and properties
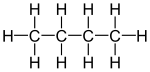 Butane is a saturated hydrocarbon gas with the chemical formula C4H10. It is an alkane with four carbon atoms. Butane is a gas at room temperature and atmospheric pressure but is easily turned into a liquid by cooling or applying moderate pressure. Butane is a safe, efficient and clean fuel that powers many standard mobile heating appliances.
Butane is a saturated hydrocarbon gas with the chemical formula C4H10. It is an alkane with four carbon atoms. Butane is a gas at room temperature and atmospheric pressure but is easily turned into a liquid by cooling or applying moderate pressure. Butane is a safe, efficient and clean fuel that powers many standard mobile heating appliances.
A far higher boiling point than propane makes butane only suitable for outdoor use during milder months. as low winter temperatures may mean the liquid will not boil to produce vapour to burn.
| Molecular formula | C4H10 |
|---|---|
| Molar mass | 58.12 g mol−1 |
| Appearance | Colourless gas |
| Odour | Odourless |
| Density | 2.48 g dm−3 (at 15 °C) |
| Melting point | -140--134 °C, 133-139 K, -220--209 °F |
| Boiling point | -1-1 °C, 272-274 K, 30-34 °F |
| Solubility in water | 61 mg L−1 (at 20 °C) |
| log P | 2.745 |
| Vapour pressure | ~25 PSI (at 50 °F) |
| kH | 11 nmol Pa−1 kg−1 |
Most butane cylinders are blue. However the butane cylinders from BP Gas, Flogas, Handy Gas & MacGas which use the 21mm butane regulator (CG4) are usually a beige/grey/gold colour whereas the butane bottles from these same suppliers which use the 20mm butane regulator (CG5) are blue. All Calor Gas butane bottles are blue and only use the CG4 regulator apart from the 4.5kg bottle which uses CG3.
| Calor Gas | MacGas | Flogas | |
|---|---|---|---|
| 4.5kg |  |
 |
|
| 7kg |  |
 |
 |
| 13kg |  |
 |
|
| 15kg |  |

| Regulator Image | Regulator Name |
|---|---|
 |
Butane 4.5 (LP) Regulator |
 |
Butane 21mm butane regulator |
 |
Butane 20mm butane regulator |
propane bottles, regulators and properties
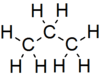 Propane is a three-carbon alkane with the molecular formula C3H8, normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central heating.
Propane is a three-carbon alkane with the molecular formula C3H8, normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central heating.
Propane is one of a group of liquefied petroleum gases. Propane should not be used indoors but because of its low freezing point it is perfect for outdoor use in the autumn and winter.
| Molecular formula | C3H8 |
|---|---|
| Molar mass | 44.10 g mol−1 |
| Appearance | Colourless gas |
| Odour | Odourless |
| Density | 2.0098 mg mL−1 (at 0 °C, 101.3 kPa) |
| Melting point | -188 °C, 85.5 K, -306 °F |
| Boiling point | -42--42 °C, 230.9-231.11 K, -44--44 °F |
| Solubility in water | 40 mg L−1 (at 0 °C) |
| log P | 2.236 |
| Vapour pressure | 853.16 kPa (at 21.1 °C) |
| kH | 15 nmol Pa−1 kg−1 |
Most propane bottles are red, but there has been a growing trend towards having green bottles for alfresco use such as barbecue gas and patio gas. Examples of these are 5kg & 10kg Mac Gaslight and the 5kg & 13kg Calor Gas Patio Gas.
Propane tends to be cheaper than butane, Butane however burns cleaner. Always ask your local stockist for advice as to which is more suitable for your use.

| Calor Gas | MacGas | Flogas | |
|---|---|---|---|
| 3.9kg |  |
 |
|
| 5kg |  |
||
| 6kg |  |
 |
 |
| 7kg |  |
 |
|
| 10kg |  |
||
| 11kg |  |
||
| 12kg *FLT |  |
 |
|
| 13kg |  |
||
| 18kg |  |
 |
 |
| 19kg |  |
 |
 |
| 47kg |  |
 |
 |